Elisa
Introduction
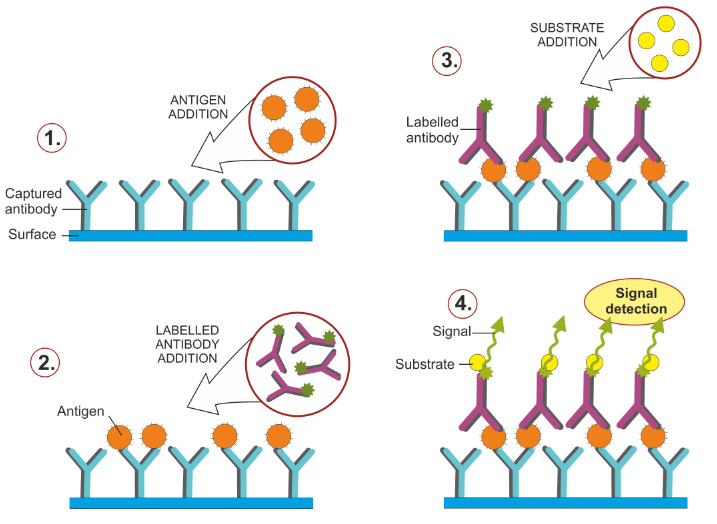
Enzyme-linked immunosorbent assay (ELISA) is a powerful method to detect and quantify a specific protein in a complex mixture. Originally described by Engvall and Perlmann (1971), the method allows the analysis of immobilized protein samples in microplate wells using specific antibodies. ELISAs are usually performed in 96- or 384-well polystyrene plates, which passively bind antibodies and proteins.
It is this binding and immobilization of reagents that makes ELISAs easy to design and perform. Having the ELISA reagents immobilized on the surface of the microplate facilitates the separation of bound from unbound material during the assay. This ability to use high-affinity antibodies and wash away non-specific bound materials makes ELISA a powerful tool for measuring specific analytes within a crude preparation. Although many variants of ELISA have been developed and used in different situations, they all rely on the same basic elements:
- Coating/Capture: Direct or indirect immobilization of antigens on the surface of polystyrene microplate wells.
- Plate Blocking: Addition of irrelevant protein or another molecule to cover all unsaturated surface binding sites in microplate wells.
- Probing/detection: incubation with antigen-specific antibodies that bind affinity to antigens.
- Signal measurement: detection of the signal generated through the direct or secondary label on the specific antibody.
The most widely used enzyme markers are horseradish peroxidase (HRP) and alkaline phosphatase (AP). Other enzymes have also been used; these include β-galactosidase, acetylcholinesterase, and catalase. A large selection of substrates is commercially available for performing ELISA with an HRP or AP conjugate. The choice of substrate depends on the assay sensitivity required and the instrumentation available for signal detection (spectrophotometer, fluorometer, or luminometer).
ELISA formats: direct, indirect and sandwich ELISA
There are several formats used for ELISA. These are classified into direct, indirect or sandwich detection and capture methods. The key step is the immobilization of the antigen of interest, either by direct adsorption to the assay plate or indirectly through a capture antibody that has been attached to the plate. The antigen is then detected directly (labelled primary antibody) or indirectly (as a labelled secondary antibody).
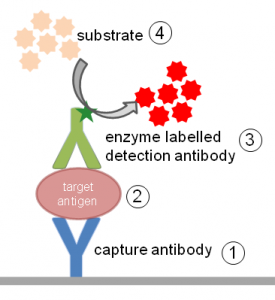
The most widely used ELISA assay format is the sandwich ELISA assay, which immobilizes and indirectly detects the presence of the target antigen. This type of capture assay is called a “sandwich” assay because the analyte to be measured is bound between two primary antibodies, each of which detects a different epitope on the antigen: the capture antibody and the detection antibody. The ELISA sandwich format is widely used due to its sensitivity and specificity.
Direct versus indirect ELISA detection strategies
Among the standard assay formats discussed and illustrated above, where differences in both capture and detection were of concern, it is important to differentiate between the particular strategies that exist specifically for the detection step. Regardless of the method by which an antigen is captured on the plate (by direct adsorption to the surface or through a pre-coated “capture” antibody, as in a sandwich ELISA), the detection step (either as direct detection or indirect) ) that largely determines the sensitivity of an ELISA.
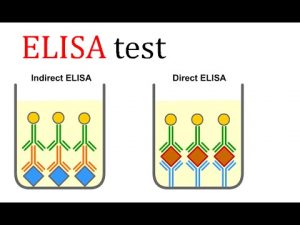
The direct detection method uses a primary antibody labelled with a reporter enzyme or a label that reacts directly with the antigen. Direct detection can be performed with an antigen that is immobilized directly on the assay plate or with the capture assay format. Direct detection, although not widely used in ELISA, is quite common for immunohistochemical staining of tissues and cells.
The indirect detection method uses a labelled secondary antibody or biotin-streptavidin complex for amplification and is the most popular format for ELISA. The secondary antibody has specificity for the primary antibody. In a sandwich ELISA, it is critical that the secondary antibody be specific for the detection of the primary antibody only (and not the capture antibody) or the assay will not be specific for the antigen. In general, this is achieved through the use of primary and capture antibodies from different host species (eg, mouse IgG and rabbit IgG, respectively).
Primary antibodies for ELISA
Monoclonal or polyclonal antibodies can be used as capture and detection antibodies in sandwich ELISA and other ELISA systems. Monoclonal antibodies have an inherent monospecificity towards a single epitope that allows accurate detection and quantification of small differences in antigen. Polyclonal antibodies are often used as a capture antibody to extract as much antigen as possible. A monoclonal is then used as the detection antibody in the sandwich assay to provide improved specificity.
In addition to the use of traditional monoclonal antibodies, recombinant monoclonal antibodies can also be used for ELISA. Recombinant antibodies are derived from antibody-producing cell lines engineered to express specific antibody heavy and light chain DNA sequences. Compared to traditional hybridoma-derived monoclonal antibodies, recombinant antibodies are not susceptible to cell line drift or lot-to-lot variation, allowing for maximal antigen specificity.
An important consideration when designing a sandwich ELISA is that the capture and detection antibodies must recognize two different epitopes that do not overlap. When the antigen binds to the capture antibody, the epitope recognized by the detection antibody should not be obscured or altered.
Capture and detection antibodies that do not interfere with each other and can bind simultaneously are called “matched pairs” and are suitable for developing a sandwich ELISA. Many primary antibody vendors provide epitope information and list antibody pairs that have been validated in ELISA as matched pairs. Using the same antibody for capture and detection may limit the dynamic range and sensitivity of the final ELISA.
Complete, ready-to-use ELISA kits
In addition to the individual components and general ELISA principles discussed in this article, ready-to-use ELISA kits are commercially available for the detection of hundreds of specific cytokines, chemokines, growth factors, neurobiological analytes, and phosphorylated proteins that are common targets of research interest.
For many lenses, there are two types of kits available:
- Coated ELISA Kits: Contains pre-coated antibody plates, detection antibodies, buffers, diluents, standards, and substrates. In addition to traditional ELISA kits, Instant ELISA kit plates are also available that contain all the necessary components, including lyophilized capture antibody and detection antibody, streptavidin-HRP, and sample diluent. In addition, strip wells containing the standard for the standard curve are provided separately to allow full use of all 96 wells for test samples.
- Uncoated ELISA Kits: These kits come with all the reagents necessary to coat your own plate and run the assay, with the exception of stop solution and wash buffer.
- Antibody Pair Kits: Contain development-optimized paired antibodies and sandwich ELISA standards (no plates or detection reagents).